Plasma
With increasing temperature, all materials are successively transformed from the solid to the liquid and then to the gaseous state. If the temperature is further increased, one gets plasma, the "fourth aggregate state of matter".
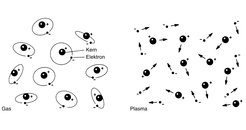
In a gas the electrons are bound to the nuclei; in a plasma the electrons have become totally or partially separated from the nuclei.
© IPP
In a plasma the atoms of the gas decompose into their constituents, electrons and nuclei. Everyday examples are the plasma column in a neon tube, an electric spark or the plasma streak of lightning.
The properties of a plasma are very different from those of ordinary gases. Plasma is, for example, electrically conductive. Its motion can therefore be influenced by electric and magnetic fields. This property is exploited in fusion devices by confining the hot plasma in a "magnetic field cage", thereby keeping it away from material walls.

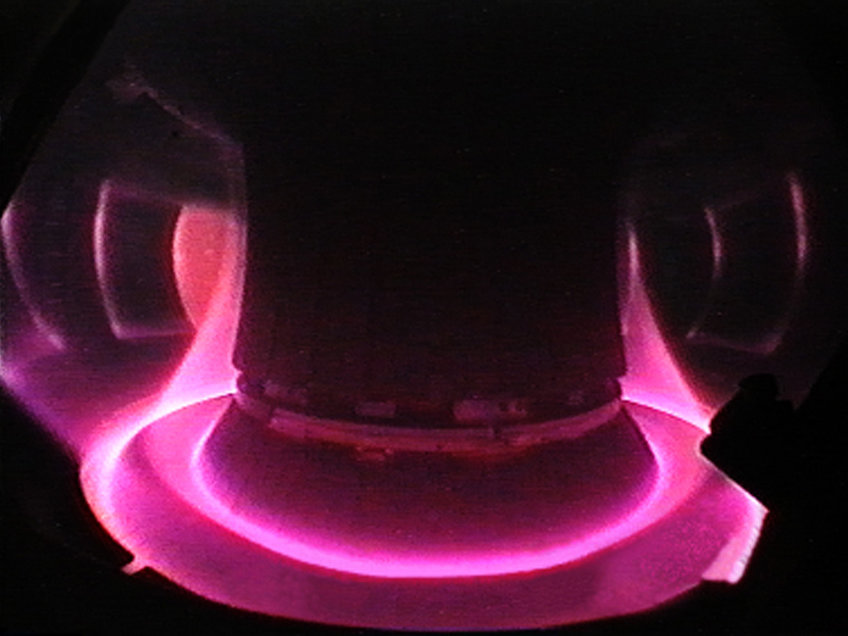
View into the plasma of the ASDEX Upgrade fusion device. The plasma shape is defined by the magnetic cage.
Photo: IPP
More plasma ...
- Ball lightning: Much discussed but still puzzling – does it consist of plasma? [more]